The Origin of Group Frequencies. Infrared radiation is emitted from the surface of Earths atmosphere at wavelengths between 400 and 700 nm and is absorbed by water vapor carbon dioxide nitrogen oxides methane ammonia nitrous oxide ozone sulfur dioxide and other gases.

Interpreting Ir Specta A Quick Guide Master Organic Chemistry Organic Chemistry Physics And Mathematics Chemical Science
Infrared IR spectra are obtained by measuring the absorption of light by molecules in the atmosphere.

. To use an IR spectrum table first find the frequency or compound in the first column depending on which type of chart you are using. How to Read an IR Spectrum Table. Look at double bond region 1600-1800 cm-1 Step 3.
Simply use a Google search of chemical name NIST EI Spectra and go to the httpwebbooknistgov option to check your work. Are any or all to the right of 3000. Then find the corresponding values for absorption appearance and other attributes.
Alkyl groups present in most organic molecules Are any or all to the left of 3000. These vibrations occur only at specific frequencies which correspond to the frequency of IR light. Begin by looking in the region from 4000-1300.
In this video we will go through some IR spectra and figure out what they are. You can pause the video practice figuring them out then unpause and hear how. Identification of a molecule by its IR spectrum even in these days is untenable.
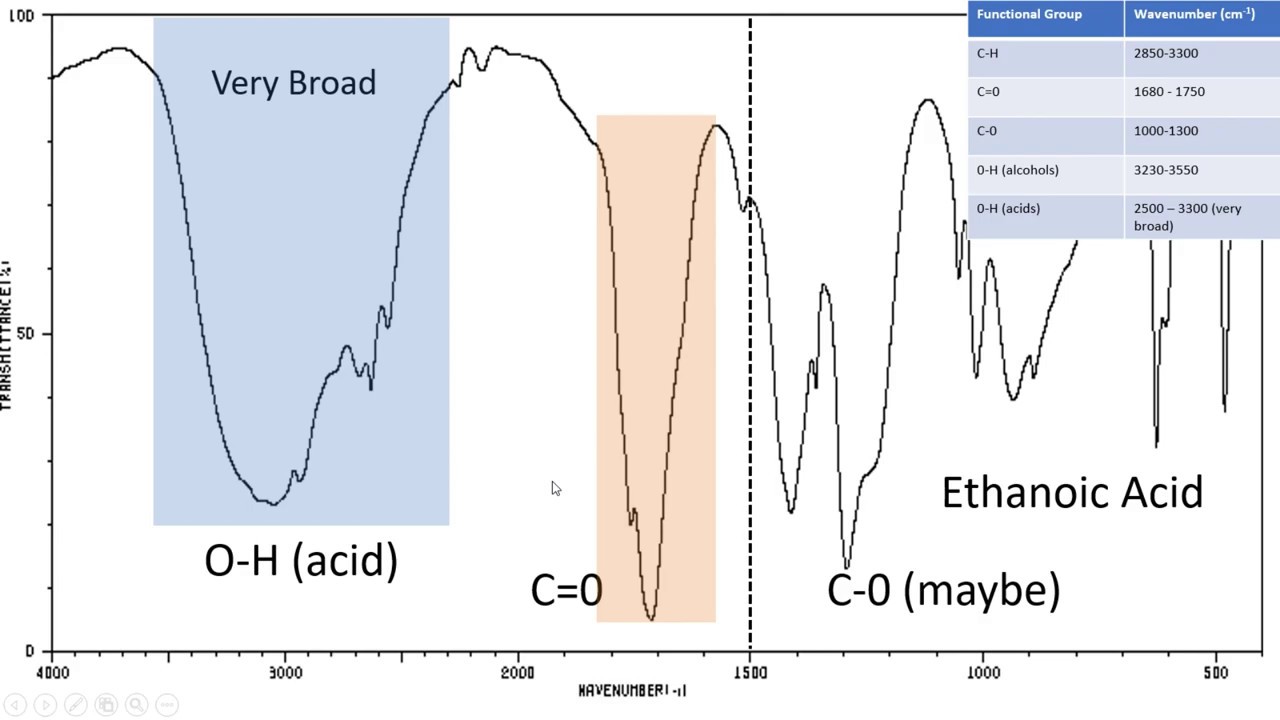
Ethanoic acid has the structure. See the links in the answer. Frequencies appear in the.
The transmittance is then plotted versus the frequency of the light which is presented in the somewhat unusual units of cm 1. Note that not all frequencies have a related compound. If your molecule contains C O or C O or N O it may be useful and it may give you an idea of molecular symmetry.
CO CN CC. The value for absorption is usually in cm-1. X-axis in units of inverse centimeters wavenumbers and intensities are plotted on the y-axis in percentage units.
When interpreting an infrared IR spectrum it can be overwhelming to take it in all at once. Same frequencies same intensities same signal shapes. This means that anyone who measures the IR spectrum of this molecule should see the same characteristic signals.
Look at the CH stretching bands around 3000. If you follow a series of steps focusing only on absorption peaks in certain regions of the IR spectrum it will be much easier to interpret. Infrared spectroscopy is a very indirect method of analysis.
If the major peaks of the NIST mass spectrum do not match your teaching spectra. When the frequency of IR light matches the frequency of a particular vibrational mode the IR light is absorbed and you can tell which frequencies are absorbed by looking at your infrared spectrum. Determine the X-axis and the Y-axis of the spectrum.
You will see that it contains the following bonds. The conventional way to plot IR spectra is with high wavenumber to the left and low wavenumber to the right as seen in Figure 5. The IR spectrum is basically a plot of transmitted or absorbed frequencies vs.
Read the Spectrum from Left to Right. An important observation made by early researchers is that many functional group absorb infrared radiation at about the same wavenumber regardless of the structure of the rest of the molecule. In infrared spectroscopy units called wavenumbers are normally used to denote different types of light.
You should read the spectrum from left to right like a sentence in a book noting the presence or absence of the group wavenumbers listed in Table I. 1 These equations show that light waves may be described by their frequency wavelength or wavenumber. Look at the diagnostic region first above 1500 cm-1 Step 2.
The X-axis of an IR spectrum is labeled as Wavenumber and ranges in number from 400 on the far right to 4000 on the far left. The Y-axis is labeled as Percent Transmittance and ranges in number from 0 on the bottom and 100 at the top. Intensity of the transmission or absorption.
A close match indicates correct identification. NH OH CH. To generate the IR spectrum different frequencies of infrared light are passed through a sample and the transmittance of light at each frequency is measured.
The infra-red spectrum for a simple carboxylic acid. The X-axis provides the absorption number. The peaks which are also called absorbance bands correspond with the various vibrations of the samples atoms when its exposed to the infrared region of the electromagnetic spectrum.
How to read IR spectra. Look at triple bond region 2100-2300 cm-1 Step 4. The signals you see in an IR spectrum are characteristic of your molecule assuming it is pure.
January 15 2022. The frequency wavelength and wavenumber are related to each other via the following equation 1. Draw a line at 3000cm-1 and observe signal above and below it.
In this video I will give you an introduction to infrared spectroscopy and explain what the graphs mean and how to interpret a spectroscopy graph. For mid-range IR the wave number on the infrared spectrum is plotted between 4000 to 400 cm-1. Different kinds of bonds vibrate at different frequencies so.
The two spectra provided and NIST standard should match closely.
Introduction To Ir Spectroscopy How To Read An Infrared Spectroscopy Graph Youtube

Interpreting Ir Specta A Quick Guide Master Organic Chemistry Organic Chemistry Organic Chemistry Study Chemistry
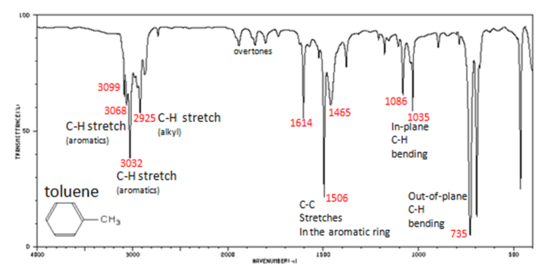
11 5 Infrared Spectra Of Some Common Functional Groups Chemistry Libretexts

How To Read Ir Spectroscopy Organic Chemistry Tutorials In This Video We Will Go Through Some I Organic Chemistry Organic Chemistry Study Teaching Chemistry
0 Comments